Seagen (SGEN) Gets Early FDA Nod for Tivdak in Cervical Cancer (Revised)
You’re reading Entrepreneur United States, an international franchise of Entrepreneur Media.
This story originally appeared on Zacks
Seagen Inc. SGEN, along with its Danish partner Genmab A/S GMAB, announced that the FDA has granted accelerated approval to their investigational antibody drug conjugate (“ADC”), Tivdak (tisotumab vedotin-tftv), for the treatment of recurrent/metastatic cervical cancer in adult patients whose disease progressed on or after chemotherapy.

– Zacks
Following the FDA nod, Tivdak became the first and only approved ADC to address the given indication.
We note that the approval of Tivdak comes before the scheduled Prescription Drug User Fee Act action date of Oct 10, 2021.
In April 2021, the FDA accepted and granted priority review to the biologics license application (“BLA”) for Tivdak. In February 2021, the BLA was submitted to the FDA for the accelerated approval of Tivdak.
The BLA was based on data from the pivotal phase II innovaTV 204 study, which evaluated Tivdak as a monotherapy for the treatment of recurrent/metastatic cervical cancer. The FDA has approved Tivdak under its Accelerated Approval Program based on tumor response and the durability of the response.
However, the approval for Tivdak came with a boxed warning for ocular toxicity.
Shares of Seagen have declined 9.2% so far this year against the industry’s rise of 0.9%.
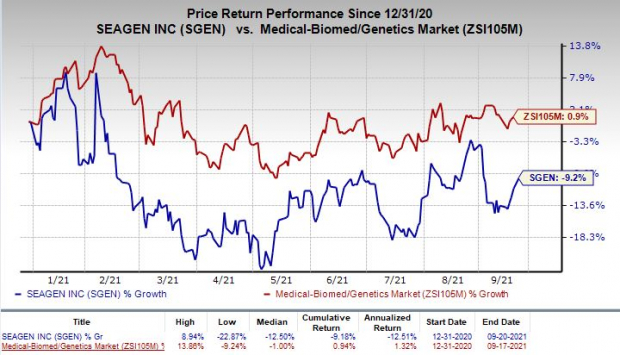
Image Source: Zacks Investment Research
Per the company, over 14,480 new cases of invasive cervical cancer are expected to be diagnosed in the United States in 2021 with 4,290 women dying from the disease. Hence, the approval for Tivdak should offer a new treatment option for the given patient population.
Seagen’s portfolio currently comprises of three marketed drugs, namely, Adcetris, Padcev and Tukysa, which are approved for different cancer indications. The company generated net product revenues of $649.9 million in the first six months of 2021, reflecting 48% growth year over year. The latest approval for Tivdak has now added a fourth drug to Seagen’s portfolio, which should drive growth for the company in 2021 and beyond.
Zacks Rank & Stocks to Consider
Seagen currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Vertex Pharmaceuticals Incorporated VRTX and Spero Therapeutics, Inc. SPRO, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Vertex’s earnings estimates have been revised 10.2% upward for 2021 and 7.3% upward for 2022 over the past 60 days.
Spero Therapeutics’ loss per share estimates have narrowed 8.2% for 2021 and 10.6% for 2022 over the past 60 days.
(We are reissuing this article to correct a mistake. The original article, issued on September 21, 2021, should no longer be relied upon.)
Infrastructure Stock Boom to Sweep America
A massive push to rebuild the crumbling U.S. infrastructure will soon be underway. It’s bipartisan, urgent, and inevitable. Trillions will be spent. Fortunes will be made.
The only question is “Will you get into the right stocks early when their growth potential is greatest?”
Zacks has released a Special Report to help you do just that, and today it’s free. Discover 7 special companies that look to gain the most from construction and repair to roads, bridges, and buildings, plus cargo hauling and energy transformation on an almost unimaginable scale.
Download FREE: How to Profit from Trillions on Spending for Infrastructure >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX): Free Stock Analysis Report
Seagen Inc. (SGEN): Free Stock Analysis Report
Spero Therapeutics, Inc. (SPRO): Free Stock Analysis Report
Genmab AS Sponsored ADR (GMAB): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
For all the latest Business News Click Here
For the latest news and updates, follow us on Google News.
