Green hydrogen: How photoelectrochemical water splitting may become competitive
Hydrogen can be produced by electrolysis of water, ideally with solar cells or wind power providing the electrical energy required. This “green” hydrogen is expected to play an important role in the energy system of the future. Over the past decade, solar water splitting has made considerable progress: the best electrolyzers, which draw the required voltage from PV modules or wind power, already achieve efficiencies of up to 30%. This is the indirect approach.
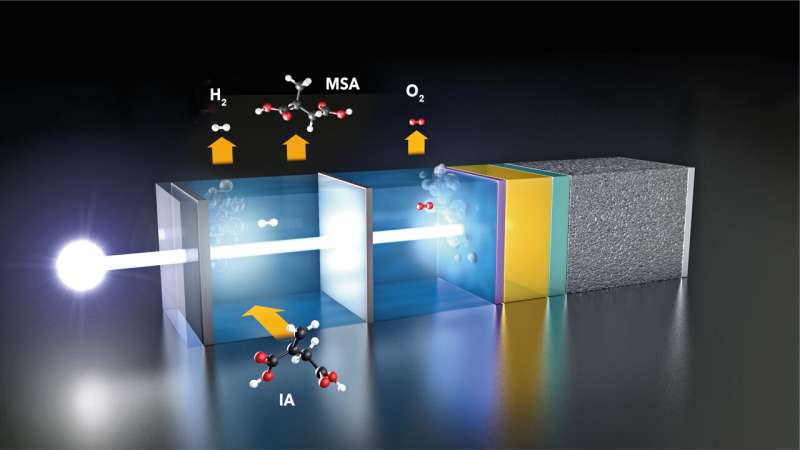
At the HZB Institute for Solar Fuels, several teams are working on a direct approach to solar water splitting: they are developing photoelectrodes that convert sunlight into electrical energy, are stable in aqueous solutions, and catalytically promote water splitting. These photoelectrodes consist of light absorbers that are intimately coupled to catalyst materials to form the active component of a photoelectrochemical cell (PEC).
The best PEC cells based on low-cost and stable metal oxide absorbers already achieve efficiencies close to 10%. Although PEC cells are still less efficient than PV-driven electrolyzers, they also have important advantages: in PEC cells, for example, the heat from sunlight can be used to further accelerate the reactions. And because current densities are ten to a hundred times lower with this approach, it is possible to use abundant and very inexpensive materials as catalysts.
Not yet competitive
So far, techno-economic analyses (TEA) and net energy assessments (NEA) have shown that the PEC approach is not yet competitive for large-scale implementation. Hydrogen from PEC systems today costs about 10 USD/kg, about 6 times more than hydrogen from fossil methane steam reforming (1.5 USD/kg). Moreover, the cumulative energy demand for PEC water splitting is estimated to be 4—20 times higher than for hydrogen production with wind turbines and electrolyzers.
“This is where we wanted to bring a new approach,” says Dr. Fatwa Abdi from the HZB Institute for Solar Fuels. Within the framework of the UniSysCat excellence network collaboration between Prof Reinhard Schomäcker and Prof Roel van de Krol, Abdi’s group investigated how the balance changes when some of the hydrogen produced reacts further with itaconic acid (IA) in the same reactor (in situ) to form methyl succinic acid (MSA).
Energy payback times
They first calculated how much energy is needed to produce the PEC cell from light absorbers, catalyst materials and other materials such as glass, and how long it has to function to produce this energy in the form of chemical energy as hydrogen or MSA. For hydrogen alone, this ‘energy payback time’ is around 17 years assuming a modest 5% solar-to-hydrogen efficiency.
If only 2% of the hydrogen produced is used to convert IA into MSA, the energy payback time is halved, and if 30% of the hydrogen is converted into MSA, the production energy can be regained after just 2 years. “This makes the process much more sustainable and competitive,” says Abdi. One reason: the energy needed to synthesize MSA in such a PEC cell is only one-seventh of the energy need of conventional MSA production processes.
“The system is flexible and can also produce other valuable chemicals that are currently needed at the site,” explains Abdi. The advantage is that the fixed components of the PEC unit, which account for most of the investment costs, remain the same; only the hydrogenation catalyst and the feedstock need to be exchanged.
“This approach offers a way to significantly reduce the production cost of green hydrogen and increases the economic feasibility of PEC technology,” Abdi says. “We have carefully thought through the process, and the next step is to test in the laboratory how well the simultaneous production of hydrogen and MSA works in practice.”
The findings are published in the journal Nature Communications.
More information:
Xinyi Zhang et al, Life cycle net energy assessment of sustainable H2 production and hydrogenation of chemicals in a coupled photoelectrochemical device, Nature Communications (2023). DOI: 10.1038/s41467-023-36574-1
Citation:
Green hydrogen: How photoelectrochemical water splitting may become competitive (2023, March 20)
retrieved 20 March 2023
from https://techxplore.com/news/2023-03-green-hydrogen-photoelectrochemical-competitive.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Technology News Click Here
For the latest news and updates, follow us on Google News.
